12.2 `Natural experiments’
It is doubtful whether so called natural experiments deserve to be called ‘experiments’ at all, and not simply well-designed field surveys, since they make comparisons of places or times which differ in the intensity of the particular environmental factor under consideration. The obvious logical flaw with this approach is that its validity rests on the assumption that places or times differ only in the intensity of the selected environmental factor (treatment); there is no possibility of randomly allocating treatments to experimental units, the central tool of experimentation and one that ensures that the potential effects of unmeasured, uncontrolled variables are averaged out across the experimental groups. Design is often a problem, but statistical techniques such as two-way ANOVA, e.g. Sokal & Rohlf (1981) , Underwood (1981) , or two-way ANOSIM (Chapter 6), may enable us to examine the treatment effect allowing for differences between sites, for example. This is illustrated in the first example below.
In some cases natural experiments may be the only possible approach for hypothesis testing in community ecology, because the attribute of community structure under consideration may result from evolutionary rather than ecological mechanisms, and we obviously cannot conduct manipulative field or laboratory experiments over evolutionary time. One example of a community attribute which may be determined by evolutionary mechanisms relates to size spectra in marine benthic communities. Several hypotheses, some complementary and some contradictory, have been invoked to explain biomass size spectra and species size distributions in the metazoan benthos, both of which have bimodal patterns in shallow temperate shelf seas. Ecological explanations involve physical constraints of the sedimentary environment, animals needing to be small enough to move between the particles (i.e. interstitial) or big enough to burrow, with an intermediate size range capable of neither ( Schwinghamer (1981) ). Evolutionary explanations invoke the optimisation of two size-related sets of reproductive and feeding traits: for example small animals (meiobenthos) have direct benthic development and can be dispersed as adults, large animals (macrobenthos) have planktonic larval development and dispersal, there being no room for compromise ( Warwick (1984) ).
To test these hypotheses we can compare situations where the causal mechanisms differ and therefore give rise to different predictions about pattern. For example, the reproductive dichotomy noted above between macrobenthos and meiobenthos breaks down in the deep-sea, in polar latitudes and in fresh water, although the physical sediment constraints in these situations will be the same as in temperate shelf seas. The evolutionary hypothesis therefore predicts that there should be a unimodal pattern in these situations, whereas the ecological hypothesis predicts that it should remain bimodal. Using these situations as natural experiments, we can therefore falsify one or the other (or both) of these hypotheses.
However, natural experiments of this kind are outwith this manual’s scope, and the chosen example concerns ecological effects of disturbance on assemblages.
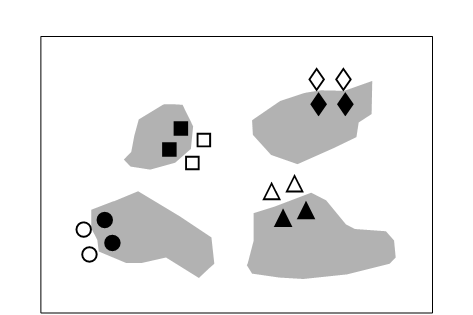
Fig. 12.1. Tasmania, Eaglehawk Neck {T}. Sketch showing the type of sample design. Sample positions (same symbols as in Fig. 12.3) in relation to disturbed sediment patches (shaded).
The effects of disturbance by soldier crabs on meiobenthic community structure {T}
On a sheltered intertidal sandflat at Eaglehawk Neck, on the Tasman Peninsula in S.E. Tasmania, the burrowing and feeding activities of the soldier crab Mictyris platycheles are evidenced as intensely disturbed areas of sediment which form discrete patches interspersed with smooth undisturbed areas. The crabs feed by manipulating sand grains in their mandibles and removing fine particulate material, but they are not predators on the meiofauna, though their feeding and burrowing activity results in intense sediment disturbance. This situation was used as a ‘natural experiment’ on the effects of disturbance on meiobenthic community structure. Meiofauna samples were collected in a spatially blocked design, such that each block comprised two disturbed and two undisturbed samples, each 5m apart (Fig. 12.1).
Table 12.1. Tasmania, Eaglehawk Neck {T}. Mean values per core sample of univariate measures for nematodes, copepods and total meiofauna (nematodes + copepods) in the disturbed and undisturbed areas. The significance levels for differences are from a two-way ANOVA, i.e. they allow for differences between blocks, although these were not significant at the 5% level.
Total individuals (N) |
Total species (S) |
Species richness (d) |
Shannon diversity (H’) |
Species evenness (J’) |
|
|---|---|---|---|---|---|
| Nematodes | |||||
| Disturbed | 205 | 14.4 | 2.6 | 1.6 | 0.58 |
Undisturbed |
200 | 20.1 | 3.7 | 2.2 | 0.74 |
| Significance (%) | 91 |
1 |
0.3 | 0.1 | 1 |
| Copepods | |||||
| Disturbed | 94 |
5.4 | 1.0 | 0.96 | 0.59 |
| Undisturbed | 146 | 5.7 |
1.0 | 0.84 | 0.49 |
| Significance (%) | 11 |
52 |
99 | 52 |
38 |
| Disturbed | 299 | 19.8 | 3.4 | 2.0 | 0.66 |
| Undisturbed | 346 | 25.9 | 4.4 | 2.3 | 0.69 |
| Significance (%) | 48 |
1 |
3 |
3 |
16 |

Univariate indices. The significance of differences between disturbed and undisturbed samples (treatments) was tested with two-way ANOVA (blocks/treatments), Table 12.1. For the nematodes, species richness, Shannon diversity and evenness were significantly reduced in disturbed as opposed to undisturbed areas, although total abundance was unaffected. For the copepods, however, there were no significant differences in any of these univariate measures.
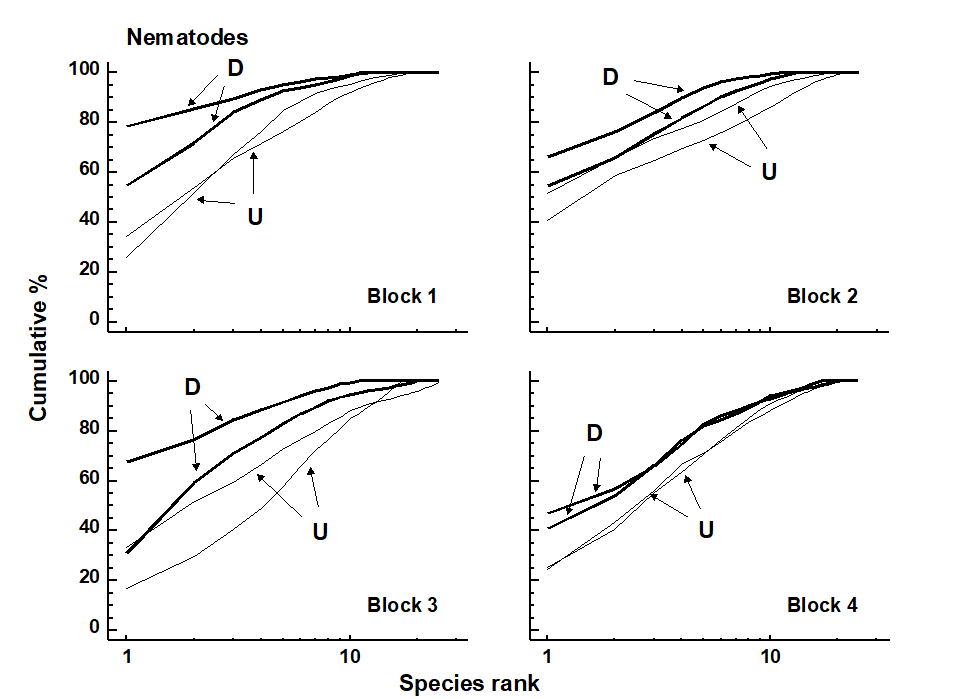
Fig. 12.2. Tasmania, Eaglehawk Neck {T}. Replicate k-dominance curves for nematode abundance in each sampling block. D = disturbed, U = undisturbed.
Graphical/distributional plots. k-dominance curves (Fig. 12.2) also revealed significant differences in the relative species abundance distributions for nematodes (using both ANOVA and ANOSIM-based tests, the latter following DOMDIS, as described on page 8.5, see
Clarke (1990)
). For the copepods, however, (plots given in Chapter 13, Fig. 13.4), k-dominance curves are intermingled and crossing, and there is no significant treatment effect.
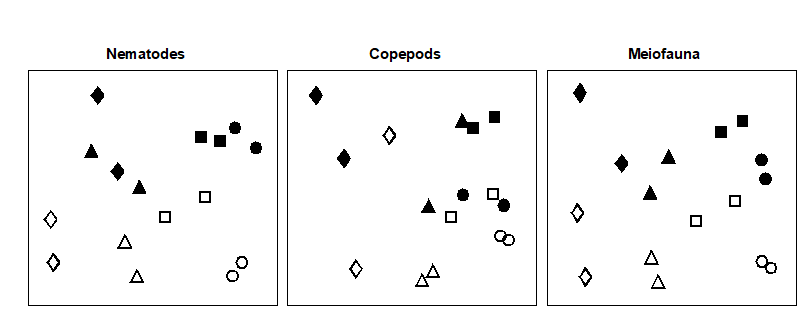
Fig. 12.3. Tasmania, Eaglehawk Neck {T}. MDS configurations for nematode, copepod and ‘meiofauna’ (nematode + copepod) abundance (root-transformed). Different shapes represent the four blocks of samples. Open symbols = undisturbed, filled = disturbed (stress = 0.12, 0.09. 0.11 respectively).
Table 12.2. Tasmania, Eaglehawk Neck {T}. Results of the two-way ANOSIM test for treatment (disturbance/no disturbance) and block effects.
| Disturbance |   Blocks Blocks |
||||
|---|---|---|---|---|---|
| R | Sig.(%) | R | Sig.(%) | ||
| Nematodes | 1.0 | 1.2 | 0.85 | 0.0 | |
| Copepods | 0.56 | 3.7 | 0.62 | 0.0 | |
| Meiofauna | 0.94 | 1.2 | 0.85 | 0.0 |
Multivariate ordinations. MDS revealed significant differences in species composition for both nematodes and copepods: the effects of crab disturbance were similar within each block and similar for nematodes and copepods. Though the ‘treatment signal’ is weaker for the latter, note the general similarities in Fig. 12.3 between the nematode and copepod configurations: both disturbed samples within each block are above both of the undisturbed samples (except for one block for the copepods), and the blocks are arranged in the same sequence across the plot. For both nematodes and copepods, two-way ANOSIM shows a significant effect of both treatment (disturbance) and blocks, Table 12.2, but the differences are more marked for the nematodes (with higher values of the R statistic).
Conclusions. Univariate indices and graphical/distributional plots were only significantly affected by crab disturbance for the nematodes. Multivariate analysis revealed a similar response for nematodes and copepods (i.e. it seems to be a more sensitive measure of community change). In multivariate analyses, natural variations in species composition across the beach (i.e. between blocks) were about as great as those between treatments within blocks, and the disturbance effect would not have been clearly evidenced without this blocked sampling design.