13.3 Macrobenthos and meiobenthos
Macrobenthos
The advantages of soft-bottom macrobenthos are that:
a) They are relatively non-mobile and are therefore useful for studying the local effects of pollutants.
b) Their taxonomy is relatively easy.
c) Quantitative sampling is relatively easy.
d) There is an extensive research literature on the effects of pollution, particularly organic enrichment, on macrobenthic communities, against which specific case-histories can be evaluated.
This combination of advantages has resulted in the soft-bottom macrobenthos being probably the most widely used component of the marine biota in environmental impact studies. Despite this, they do have several disadvantages:
a) Relatively large-volume sediment samples must be collected, so that sampling requires relatively large research ships.
b) Because it is generally not practicable to bring large volumes of sediment back to the laboratory for processing, sieving must be done at sea and is rather labour intensive and time consuming (therefore expensive).
c) The potential response time of the macrobenthos to a pollution event is slow. Their generation times are measured in years, so that although losses of species due to pollution may take immediate effect, the colonisation of new species which may take advantage of the changed conditions is slow. Thus, the full establishment of a community characterising the new environmental conditions may take several years.
d) The macrobenthos are generally unsuitable for causality experiments in mesocosms, because such experiments can rarely be run long enough for fully representative community changes to occur, and recruitment of species to mesocosm systems is often a problem because of their planktonic larval stages (see Chapter 12).
Example: Amoco Cadiz oil-spill
The sensitivity of macrobenthic community structure to pollution events, when using multivariate methods of data analysis, is discussed in Chapter 14. The response of the macrobenthos in the Bay of Morlaix to the Amoco Cadiz oil-spill some 40 km away, already seen in Chapters 5, 7 and 10, is a good example of this (Fig. 13.3).
Fig. 13.3. Amoco-Cadiz oil spill, Bay of Morlaix {A}. MDS for macrobenthos at station ‘Pierre Noire’, at approximately three-monthly sampling intervals (stress = 0.09).
Meiobenthos
Apart from sharing the advantage of non-mobility, and therefore usefulness for local effects studies, the relative advantages and disadvantages of the meiobenthos are exactly the reverse of the macrobenthos. Their advantages are:
a) Because of their small size and high density in marine sediments, quantitative sampling of the meiobenthos is easy from small ships, open boats etc.
b) The small volume of the samples means that they can easily be transported to the laboratory, and need not be processed on board ship.
c) Their generation times are usually measured in months rather than years, so that their potential response time to pollution events is much faster than that of the macrobenthos.
d) Because of this fast response time, and direct benthic rather than planktonic development, the meiobenthos are good candidates for causality experiments in experimental microcosms and mesocosms.
The disadvantages of meiobenthos are that:
a) Their taxonomy is considered difficult. Identification of almost all the meiobenthic taxa to species level presents difficulties even in Europe and N America, and in many parts of the world the fauna is almost completely unknown. However, to a considerable degree, three factors mitigate against this problem:
i. The robustness of community analyses to the use of taxonomic levels higher than species (see Chapter 10).
ii. The cosmopolitan nature of most meiobenthic genera.
iii. The increasing availability of easily used keys to meiobenthic genera. For example, the pictorial keys to marine nematodes of Platt & Warwick (1988) have been used successfully worldwide.
b) Community responses of the meiobenthos to pollution are not as well documented as for the macrobenthos, and there is only a modest body of information in the literature against which particular case-histories can be evaluated.
Example: Soldier crab disturbance of nematode assemblages, Tasmania
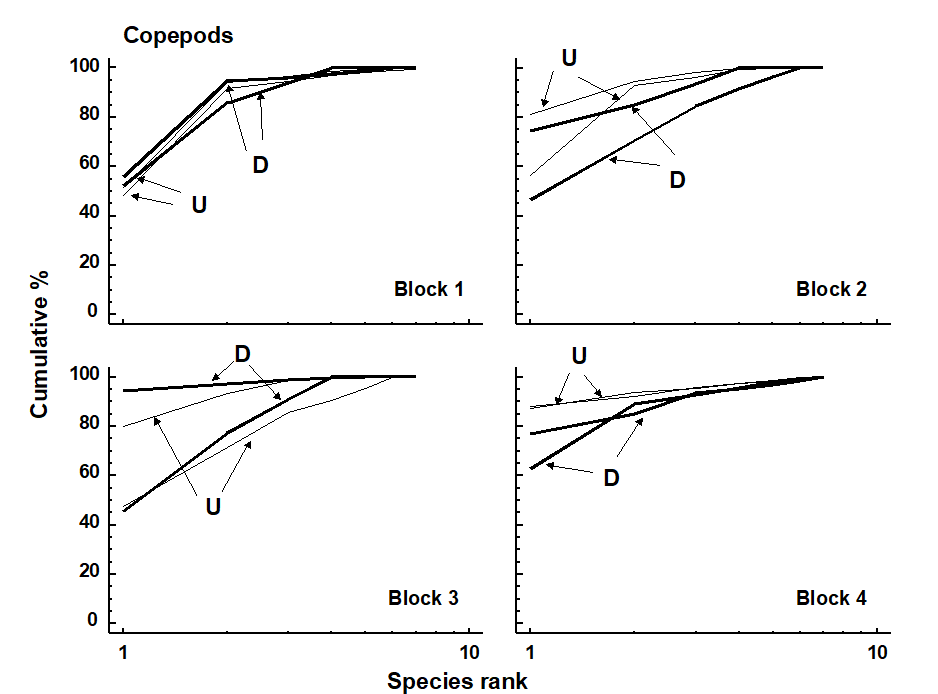
This natural field experiment was first met in Chapters 6 and 12. It will be remembered that the nematode diversity profiles were affected by the crab disturbance (Fig. 12.2), whereas no significant effect was noted for copepods (Fig. 13.4). Many nematode species are more sedentary in habit than copepods, often adhering to sand-grains by secretions from their caudal glands, and some species prefer conditions of low oxygen concentration or are obligate anaerobes. The so called ‘thiobiotic’ meiofaunal community contains many nematode species, but apparently no copepods. Non-bioturbated sediments will have a vertical gradient in physical and chemical conditions ranging from wave-disturbed sediments with an oxiphilic meiofauna community near the surface to a stable sediment with a thiobiotic community deeper down. Dramatic disturbance by crabs, of the kind found at this site, will inevitably destroy this gradient, so that the whole sediment column will be well aerated and unstable. This reduction in habitat complexity is probably the most parsimonious explanation for the reduction in nematode species diversity.
Fig. 13.4. Tasmania, Eaglehawk Neck {T}. k-dominance curves for disturbed (D) and undisturbed (U) copepod samples in in 4 separate sampling blocks.
The differential response of these two components of the meiobenthos has been elaborated here in order to demonstrate how a knowledge of the biology of these components can aid in the interpretation of community responses to perturbation. The macrobenthos and meiobenthos may also respond differently to different kinds of perturbation (e.g. physical disturbance, pollution) so that a comparative study of both may be indicative of the cause.
Example: Macrobenthos and meiobenthos in Hamilton Harbour, Bermuda
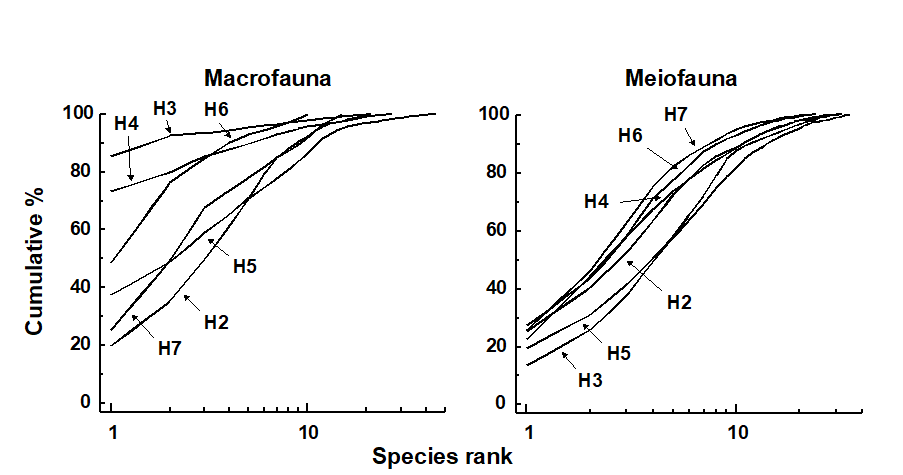
Fig. 13.5 shows the average k-dominance curves for the macrobenthos and the nematode component of the meiobenthos at six stations in Hamilton Harbour. For the macrobenthos, the curves at three of the stations (H3, H4 & H6) are much more elevated than the other three, suggesting some kind of perturbation at these sites. For the nematodes, however, all curves are closely coincident. There must therefore be some form of perturbation affecting the macrobenthos but not the meiobenthos, and it was suggested by Warwick, Platt, Clarke et al. (1990) that this is more likely to be physical disturbance of the sediment resulting from the regular passage of large cruise liners within the harbour, rather than pollution. This is because the macrobenthos are much more dependent on sediment stability to maintain diversity than are the meiobenthos.
Fig. 13.5. Hamilton Harbour, Bermuda {H}. k-dominance curves for macrobenthos (left) and meiobenthic nematodes (right) at six stations (H2-H7).